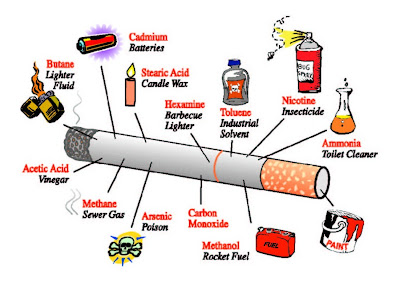
We all brush our teeth daily. But do you all know the important of brushing your teeth with toothpaste???

Toothpaste is a paste or gel dentifrice used with toothbrush. Toothpaste and a correct brushing action work to remove plaque, a sticky, harmful film of bacteria that grows on your teeth that causes caries, gum disease, and eventual tooth loss if not controlled. Toothpaste contains fluoride, which makes the entire tooth structure more resistant to decay and promotes remineralisation, which aids in repairing early decay before the damage can even be seen. Special ingredients in the dentifrice help to clean and polish the teeth and remove stains over time and toothpaste also help freshen breath and leave your mouth with a clean feeling.
Do we really concern about the ingredient of the toothpaste that we use in our daily life??? Do we??? Next time when you go to brush your teeth, take a good look at the label on the toothpaste. If you're like most people, you know your toothpaste contains fluoride. But take a good long look at the inactive ingredients. While you may find one active ingredient, you may find a dozen or more inactive ingredients on your toothpaste.
We all know the active ingredient in toothpaste typically its sodium fluoride. But sodium fluoride makes up less than one percent of toothpaste ingredients. What's the other 99% of toothpaste made up of? If most of what goes into toothpaste is labelled as inactive ingredients, why are these chemicals there? And what exactly are you putting in your mouth? The answers may surprise you.

Active Ingredients
Active ingredients are the chemically active portion of a product that works to relieve your symptoms. In toothpaste the active ingredients are what help to protect and strengthen your teeth. However, different toothpastes contain different functions, and thus may have differing active ingredients. For example, while ordinary toothpaste may only contain sodium fluoride as an active ingredient, tartar control toothpaste may also contain triclosan as an anti-gingivitis agent.
Sodium Flouride
A typical percentage of sodium fluoride found in toothpaste is 24%. Sodium fluoride is thought to strengthen teeth through the formation of flouroapatite, a component of tooth enamel. Besides toothpaste, sodium fluoride was used in the production of ceramics and rat poison. Hah???
Triclosan
Triclosan is an antibacterial and antifungal organic compound found in many antibacterial soaps, deodorants, acne medications, and other antibacterial applications. Some studies have shown that the popular Microban treatment you can find embedded into any number of hard surfaces contains Triclosan.
Inactive Ingredients
Okay, now we see what's the other 99% of stuff you put in your mouth when brushing your teeth?
Hydrated Silica
This is the abrasive used to polish and scrub the surface of your teeth in gel toothpastes. If you dry out hydrated silica you get the common silica gel found inside pepperoni packages and electronics. Silica gel absorbs water, so it is placed in little packets (coincidentally labelled, "Do Not Eat") inside packages of electronics and other items susceptible to damage from high humidity or water condensation. The water absorbing ability of silica gel also makes it a perfect ingredient for cat litter.
Sorbitol
After water, this is one of the top ingredients in children's toothpastes and is one of the main ingredients in adult toothpastes as well. So why is sorbitol more prevalent in children's toothpaste? Sorbitol is a form of most children's favourite ingredient sugar. Also known as glucitol, sorbitol is formed by reducing glucose into a form called sugar alcohol that retains 60% of sugar's sweetness with one-third the calories.
Sorbitol's use in toothpaste is three-fold. As a sweetener, it makes gives toothpaste a pleasant taste. It's also humectants, helping toothpaste stay moist. As an emulsifier, sorbitol works to keep the ingredients in toothpaste from "shifting". Ever notice that you don't have to shake a tube of toothpaste to mix it up? An emulsifier keeps the ingredients mixed up. Sorbitol was the ingredient that prompted this article. I noticed it was the main ingredient in light pancake syrup just shortly after noticing in children's toothpaste listed sorbitol as a primary ingredient. So children's is brushing their teeth with pancake syrup? Sorbitol is also used in sugar free mints, diet drinks, light pancake syrup (obviously) and as a skin softener in some soaps.
Glycerine
Another sugar alcohol used as a sweetener and humectants, glycerine makes toothpaste sweeter and smoother. It's found in a variety of products including cough syrup, shaving cream, and hair care products. In other forms glycerine is used to manufacture margarine and nitro-glycerine.
PVM/MA Copolymer
PVM/MA copolymer is a water fixative found in hairspray that does a wonderful job of making triclosan stick to your oral tissues longer. In other words, it helps triclosan stay on your teeth and gums where it can kill bacteria, rather than getting rinsed away.
Sodium Laurel Sulfate
You can find this detergent in just about any soap or shampoo (or toothpaste) that creates lots of suds. When you brush your teeth and the foam starts to appear, you can thank sodium laurel sulfate (SLS). Some studies link SLS to mouth ulcers.
PEG-12 (Dimethicone Copolyol)
This is a water-soluble polyether-modified silicone commonly used in hair conditioners.
Tetrasodium pyrophosphate
Used as a buffering and thickening agent in a variety of foods including marshmallows, pudding, chicken nuggets, and soy-based meat alternatives. It's a mildly toxic clear compound that acts as a tartar control compound by removing calcium and magnesium from teeth. It is occasionally found in household detergents to remove these minerals from clothing as well.
Cocamidopropyl Betaine
This is a special kind of detergent that acts as an acid or a base. It is non-irritating cleanser used in shampoos and its anti-static properties are used in conditioners. In its pure form it is a pale yellow liquid.
Propylene Glycol
Other humectants used to keep toothpaste from drying out, propylene glycol is the main ingredient in many shampoos, deodorants and hair dye. It's also used in smoke machines to make fake smoke and as an ingredient in deicing fluid for airplanes.
Cellulose Gum
This chemically inert thickener is used to help maintain toothpaste texture.
Titanium Dioxide
Typically used to colour your toothpaste a brilliant white, titanium dioxide is also used provide opacity. It can also be found in sunscreens, paint, plastics and pills.
CarrageenanThis is another thickener and stabilizer used in toothpaste. Carrageenan has the unique ability to thin out under stress and then bounce back when the stress is no longer applied. In other words, they help make toothpaste easy to squeeze out of the tube, but help it stiffen back up on the toothbrush. This ingredient comes from seaweed and is used in dozens of applications from air-freshener gels to processed meats and ice cream.
FD&C Blue Number 1
This synthetic dye is made from coal tar and is used to colour toothpaste, soap, shampoo and food. It's estimated that the average American consumes 16 mg of this dye a day.
Obviously not all toothpaste manufacturers’ use the exact same ingredients, but these are the main ingredients used in most toothpastes and a surprising number of other personal hygiene products. So whether you are using Colgate, Crest, Aquafresh, Rembrandt or whatever your favourite toothpaste happens to be, take a look at the inactive ingredients and check out exactly you're putting in your mouth the results may surprise you.












 Another picture of ice cream to give you strength to continue reading =P
Another picture of ice cream to give you strength to continue reading =P











 A cationic detergent is most likely to be found in a shampoo or clothes "rinse". The purpose is to neutralize the static electrical charges from residual anionic (negative ions) detergent molecules. Since the negative charges repel each other, the positive cationic detergent neutralizes this charge.
A cationic detergent is most likely to be found in a shampoo or clothes "rinse". The purpose is to neutralize the static electrical charges from residual anionic (negative ions) detergent molecules. Since the negative charges repel each other, the positive cationic detergent neutralizes this charge.





 It acts as a diuretic it increases the rate of bodily urine excretion, and delays fatigue -having the effect of warding off drowsiness and restoring alertness. Caffeine is probably the most popular drug in use because of these reasons. The effects that we might notice after consuming a large cup of coffee are hands getting cold, muscles tensing up, feeling of excitement and increased heart beat.
It acts as a diuretic it increases the rate of bodily urine excretion, and delays fatigue -having the effect of warding off drowsiness and restoring alertness. Caffeine is probably the most popular drug in use because of these reasons. The effects that we might notice after consuming a large cup of coffee are hands getting cold, muscles tensing up, feeling of excitement and increased heart beat.
 producing an excess of blood in the head area (not necessarily on the brain), and leading to a headache often lasting several days. Other withdrawal symptoms reported are fatigue and muscle pain, irritability, inability to work, nervousness, restlessness, and feeling sleepy, and in extreme cases, nausea and vomiting.
producing an excess of blood in the head area (not necessarily on the brain), and leading to a headache often lasting several days. Other withdrawal symptoms reported are fatigue and muscle pain, irritability, inability to work, nervousness, restlessness, and feeling sleepy, and in extreme cases, nausea and vomiting. 
 When you slice through an onion, you also cut through the onion cells. When the cells are cut, enzymes which are normally inside the cell are released. One of these enzymes, alliinase (from the onion), then reacts with a sulphur-containing compound known as ‘Prensco', that is also released by cutting the cells. This reaction results in the formation of 1-propenylsulphenic acid, which is further converted by the LF-synthase enzyme to propanethial S-oxide gas. This gas is also known as the Lachrymatory Factor (‘crying factor'), which also explains the name of the enzyme LF-synthase (meaning Lachrymatory Factor synthesising enzyme)
When you slice through an onion, you also cut through the onion cells. When the cells are cut, enzymes which are normally inside the cell are released. One of these enzymes, alliinase (from the onion), then reacts with a sulphur-containing compound known as ‘Prensco', that is also released by cutting the cells. This reaction results in the formation of 1-propenylsulphenic acid, which is further converted by the LF-synthase enzyme to propanethial S-oxide gas. This gas is also known as the Lachrymatory Factor (‘crying factor'), which also explains the name of the enzyme LF-synthase (meaning Lachrymatory Factor synthesising enzyme)